
 Center
Center
Explaining and presenting the basics of clinical research to scientists: a key mission of the Center for Translational Science
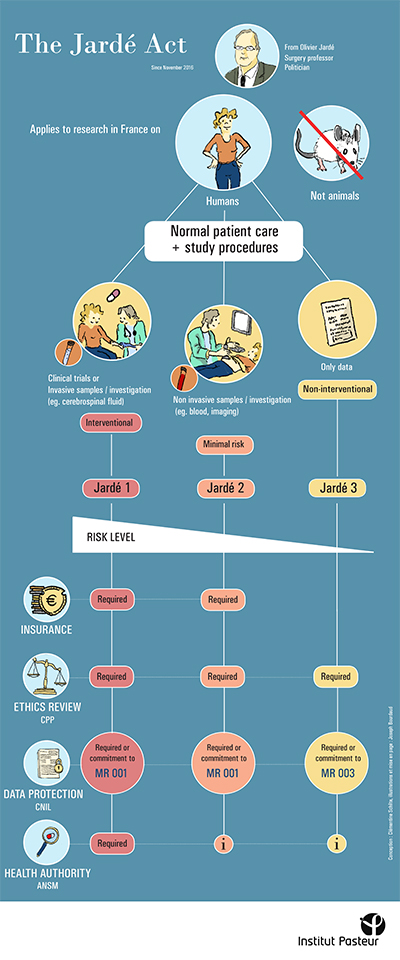
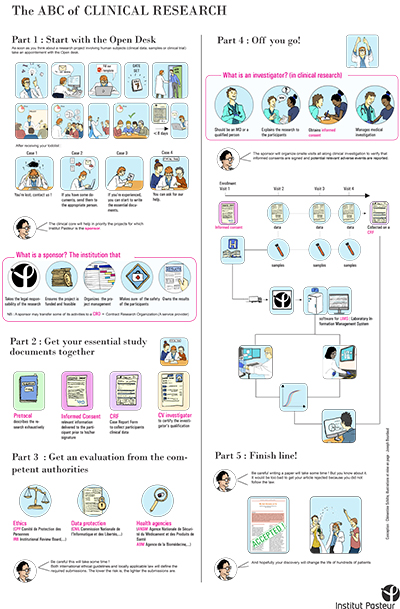
As the 9th edition of the report on "France's attractiveness for clinical research" is published by Leem (the French Association of Pharmaceutical Companies), the Center for Translational Science would like to take the opportunity to remind staff of the procedures required for research involving human subjects (samples, clinical data and clinical trials).
In an effort to communicate as clearly as possible about this important subject, the Center has decided to adopt an original approach, working in collaboration with an intern in scientific illustration from the prestigious École Estienne design school, Joseph Bourdaud, who has produced the following posters:
A poster "The ABC of clinical research", which provides a basic overview of clinical research involving human subjects at the Institut Pasteur. The poster particularly highlights the importance of attending the Center's "open desk" sessions as far in advance as possible.
The Center for Translational Science would like to remind you that if any of your projects involve human samples or data, it is important to come and present them at one of the Center's "open desk" sessions. These sessions take place every Thursday (registration required) and give you the opportunity to make contact with all the support services you will need to work with for your project, so that you can anticipate the procedures required (register by emailing crt-opendesk@pasteur.fr).
- Clinical trials: 9th report on "France's attractiveness for clinical research"
This report, commissioned by Leem (the French Association of Pharmaceutical Companies), provides an inventory of all research conducted by French pharmaceutical companies between January 1, 2016 and December 31, 2017. It has been carried out every two years since 2002 and is a way of monitoring and assessing France's position in a competitive international context and highlighting strengths and weaknesses so as to pinpoint areas where progress is needed. This year, the Leem report also contains an international comparison based on an analysis of the clinicaltrials.gov database, in addition to the survey carried out with pharmaceutical companies in France.
Main findings:
• Industry players are the main funders of clinical trials
• France is currently in fourth position in Europe for participation in new industrial clinical trials; its participation has fallen by an average of 13% each year
• French participation in new phase 1 and 2 industrial trials worldwide is low (6% and 13% respectively)
• A period of nearly 7 months is needed between the first request for approval and the inclusion of the first patient (6 months with the EU regulation pilot phase)
• France has more than 3,300 centers where clinical trials are conducted and French investigators meet 85% of their recruitment targets (100% in oncology)
• Oncology is the primary therapeutic area for clinical trials in France (19% participation in new industrial trials worldwide; 45% of clinical trials in France are in this field)
The results can be consulted in full.