 Research
Research
Jardé Law: A new framework for research involving human subjects in France
Several research projects involving human subjects are conducted on campus. The research in question can involve healthy volunteers or patients, or be based on human biological samples or on personal data.
Research on human subjects must comply with the legal and regulatory framework to ensure protection of the subjects used. The Institut Pasteur Legal Affairs Department - with its in-house staff, stakeholders and experts working in the field of research on human subjects - endeavors to ensure that these principles are followed.
To this end, The Institut Pasteur Legal Department would like to inform you that the Jardé Law of March 5, 2012 on research involving human subjects came into force on November 17, 2016.
This new law introduces significant changes to the main categories of research involving human subjects, which are now assessed on the basis of the level of risk and burden for individuals participating in research.
- Who is affected?
This law will have an impact on all scientists wishing to conduct a research project involving human subjects, the collection of human biological samples and/or personal health data.
- What changes does the Jardé Law introduce?
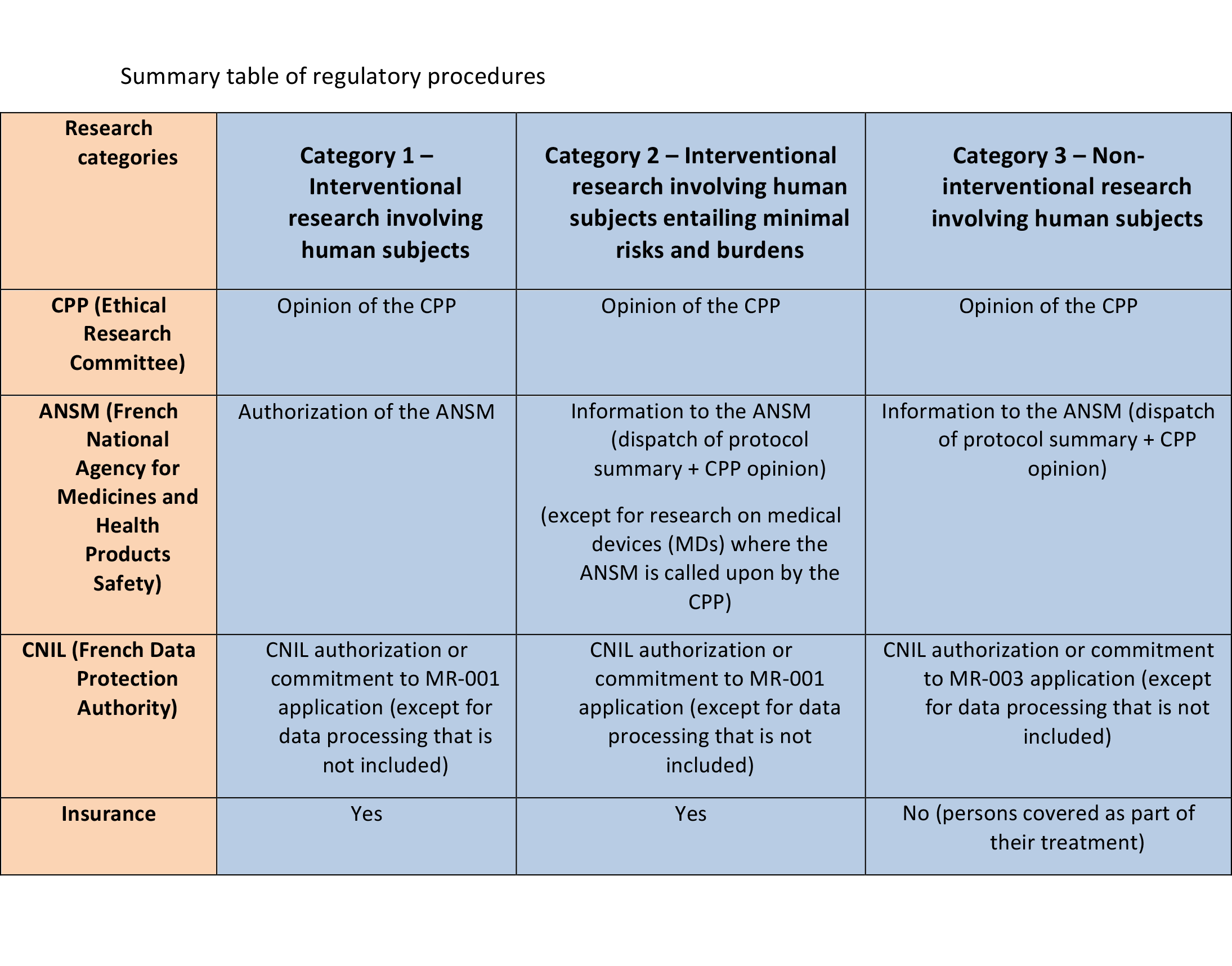
1- A new classification system for research: there are three categories depending on the risks and burdens associated with the research.
2- New regulatory procedures: the opinion of an Ethical Research Committee (CPP) is now required for all research involving human subjects (*link to summary table on regulatory procedures).
3- A new research framework for retrospective health data, with new responsibilities for the CEREES (Expert Committee for Research and Evaluation in the Field of Health) in this area.
- What criteria are used to classify a clinical research protocol?
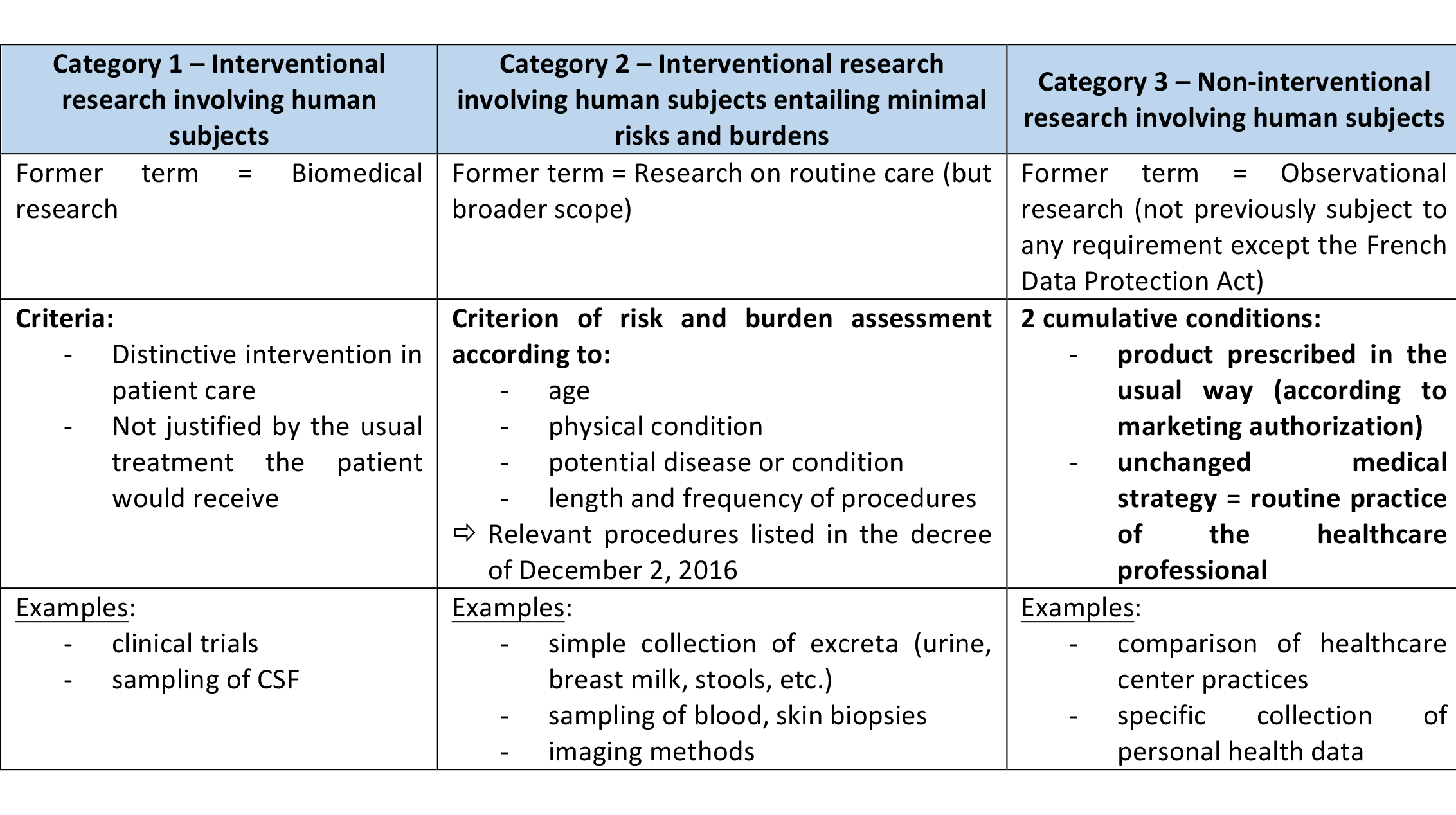
Classifying a research protocol requires a distinction to be made between treatment and research.
When writing the protocol, it is important to identify procedures that are part of the treatment and procedures that are added by research.
Under the new criteria laid down by the Jardé Law, four questions are therefore essential:
- What procedures are added by the research element?
- Do these procedures alter the usual treatment the patient would receive?
- Is the risk added by these procedures minimal?
Check if the procedures associated with research are on the list of procedures with minimal risk and burden, as provided by the decree of December 2, 2016.
- Is the aim of the research to evaluate medication?
According to the answers to these questions, the protocol will come under one of the 3 research categories provided for by the Jardé Law:
- Find out more
The Legal Department ran three training sessions in February for those particularly affected by this new law (Clinical Core of the Center for Translational Science, ICAReB platform, members of the Clinical Research Committee, etc.). The Health Center is available to organize further training if required.
The aim is to inform as many people as possible about the new regulations introduced by the Jardé Law so that those whose work is affected will understand the scope and the practical consequences for research involving human subjects and will be given support and guidance on how to comply with regulatory procedures.
- Contact
Legal Department (Anne-Laure Morin, Berengère Menu, Cloé Giquel): dj-sante@pasteur.fr