
June 10, 2016
Bulletin interne de l'Institut Pasteur

Identification of a therapeutic compound for an autistic disorder of genetic origins thanks to high-throughput screening
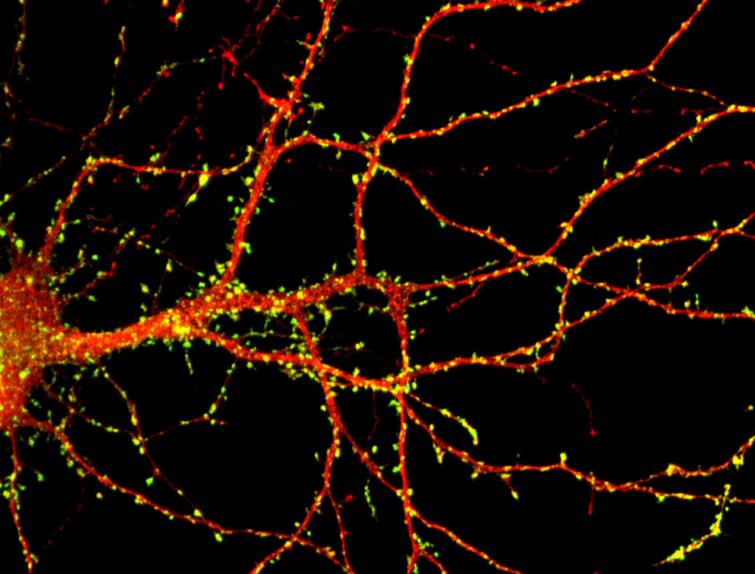
A team of researchers from I-STEM laboratory (CECS / AFM-Telethon / Inserm), led by Alexandra Benchoua and Marc Peschanski, in collaboration with Prof. Thomas Bourgeron (Pasteur Institute / Paris Diderot University / CNRS) and Prof. Richard Delorme (Robert Debré Hospital / AP-HP), has highlighted the therapeutic potential of lithium in a patient with a rare form of autism spectrum disorder associated with SHANK3 gene mutation. This molecule, usually used to treat bipolar disorder, was identified thanks to high throughput screening of chemical compounds on human neurons derived from pluripotent stem cells, including those of the treated patient. This study is a first step towards the development of tools for a more personalized medicine for individuals with autism spectrum disorders. This work was published May 27, 2016 in EBioMedicine review.
Autism spectrum disorders (ASD) represent a heterogeneous group of neurodevelopmental disorders that affect 1% of the population. They represent a challenge for the development of effective drug therapies because of the symptom complexity and variability between individuals. For about one third of patients with autism, a genetic cause has been identified. It was estimated than 1 to 2% of children with autism and intellectual disability are deficient for SHANK3 gene, a gene involved in Phelan-McDermid syndrome (the vast majority of patients with this syndrome have lost a fragment of chromosome 22 carrying a copy of SHANK3). It is to this population that was addressed the study. The loss of one of two copies of SHANK3 causes a decrease in the amount of SHANK3 protein and impairs the maintenance of the contact points between neurons (synapses). The aim of the researchers was therefore to identify drugs that can increase SHANK3 expression by activating the second still functional copy. However, SHANK3 being expressed only in neurons, it was first necessary to overcome a technological barrier to produce in vitro human neurons carrying the mutations of patients awaiting a treatment.