 News
News
A new approach to developing mouse 'embryos' in vitro
The study of embryonic development is a complex and tightly regulated field. By influencing a process that regulates gene expression, researchers at the Institut Pasteur have succeeded in recreating in vitro mouse cell clusters that mimic in some respects the structure of real embryos. By avoiding the manipulation of embryos, these approaches pave the way for applications such as the study of cancers, congenital diseases and infertility, and could provide a source of tissue for regenerative medicine. The reconstruction of in vitro embryo models is considered, according to Nature journal, as one of the emerging technologies that should revolutionize science in the coming years (1).
An embryo is an organism in the early stages of development, from the formation of the egg after fertilization to the point at which the major organs are formed. This period of embryo development, which lasts about 8 weeks in humans and will lead to the fetus, includes a succession of decisive stages with the process of Cleavage (first division of the cells in the early development of the embryo), gastrulation (installation of the three germ layers) and organogenesis (formation of internal organs).
Research involving human embryos is highly regulated and governed by strict bioethics rules. It has been authorized in France since 2013 under certain conditions and is controlled by the national Biomedicine Agency (more on embryo and embryonic stem cell research). For example, in many countries, it is prohibited to grow and use human embryos beyond 14 days after fertilization. In compliance with these ethical regulations, Institut Pasteur scientists are developing in vitro research tools that make it possible to avoid the manipulation of embryos.
A promising synthetic embryo technology
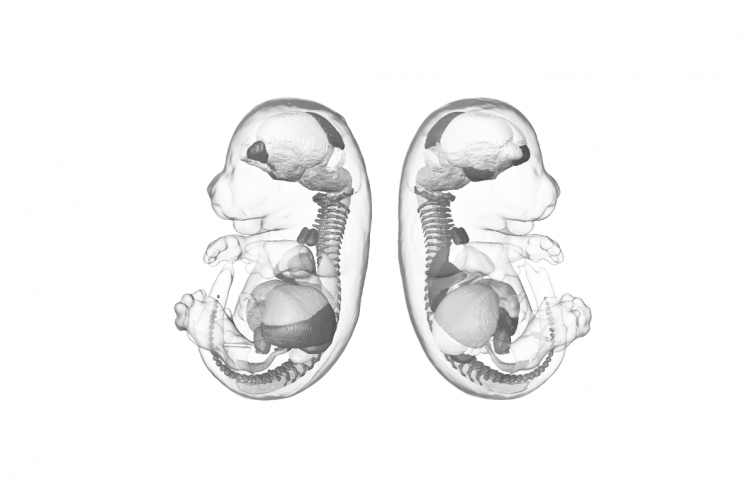
To understand the fundamental processes involved in embryonic development, the researchers focused their studies on other mammals, notably mice. Although enormous progress has been made in recent decades, our understanding of the molecular mechanisms involved in the emergence and organization of cell types within the embryo remains limited. This is due to the scarcity of biological material and the complexity of studying embryos in utero, which makes a dynamic approach to embryogenesis difficult. To overcome these limitations, synthetic embryos have been developed in vitro.
Two other laboratories recently made a major breakthrough. They managed to reconstruct, from embryonic stem cells, synthetic embryos that almost perfectly mimic the development of the mouse up to the fetal stage (2,3). But the procedure is complex and involves sophisticated equipment, with a success rate of less than 1%. These constraints do not make it sufficiently reproducible for large-scale applications.
An improved approach for developing mouse embryos in vitro
Scientists from the Institut Pasteur, Inserm and the École Polytechnique, in collaboration with the Helmholtz Center and the University of Copenhagen, have developed a new approach for generating synthetic mouse embryos (4). It consists solely of treating mouse embryonic stem cells with a chemical molecule for a short period of time. This inhibits the attachment of a small protein, called SUMO, to other proteins in the cell. By acting on the protein complexes of chromatin (the structure in which DNA is packaged), SUMO ensures stability of gene expression, which gives the cell its identity: this phenomenon is called SUMOylation.
The Institut Pasteur and Inserm Nuclear Organization and Oncogenesis Unit has been studying SUMOylation for a long time. "We hypothesized that embryonic stem cells could enter an unstable state if deficient for SUMOylation and placed in a favorable environment, with SUMO no longer acting as the gatekeeper of cellular identity. This reset would then be conducive to the emergence of cell types present in the early natural mouse embryo," explains Anne Dejean, head of the unit and co-author of the study.
An efficient and user-friendly model
To test this theory, scientists exposed mouse embryonic stem cells to a molecule that inhibits action of the SUMO protein. "We were very surprised to find not only that many differentiated cell types emerged, but that they were capable of spontaneous and harmonious self-organization, ultimately forming a polarized structure that mimicked the main characteristics of a mouse embryo," said Jack-Christophe Cossec, a unit researcher and co-author of the study.
Although the overall architecture is less advanced and the cellular diversity less extensive than in the other models, three quarters of the structures generated form pseudo-embryos that progressively elongate to form a head and a trunk (see video below).
Moreover, the use of a microfluidic chip developed in the Institut Pasteur's Physical Microfluidics and Bioengineering Unit makes the experiment much more reproducible.

We knew that it was possible to return mature differentiated cells to their stem cell state by targeting the chromatin. This study proves that the opposite may be true: by intervening on the stabilization of protein complexes at the chromatin level, it is possible to generate a range of differentiated cells that assemble correctly to form a pseudo-embryo.
The efficiency and simplicity of this approach open up the possibility of using molecules to regulate the chromatin state, in the hope of one day reconstructing exact models of organs and embryos. These models could lead to a better understanding of the mechanisms that orchestrate the establishment of cellular identity and dysfunction, particularly in tumor transformation.
This study comes under the Cancer Initiative in the Institut Pasteur's 2019-2023 Strategic Plan.
Source :
1. Eisenstein, M. Seven technologies to watch in 2023. Nature 613, 794–797 (2023).
2. Amadei, G. et al. Synthetic embryos complete gastrulation to neurulation and organogenesis. Nature (2022) doi:10.1038/s41586-022-05246-3.
3. Tarazi, S. et al. Post-gastrulation synthetic embryos generated ex utero from mouse naive ESCs. Cell 185, 3290-3306.e25 (2022).
4. Cossec, J.-C. et al. Transient suppression of SUMOylation in embryonic stem cells generates embryo-like structures. Cell Reports (2023).